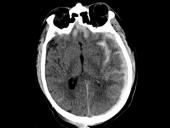
BARDA is partnering with Abbott to broaden the company’s current traumatic brain injury (TBI) test that will aid healthcare providers in diagnosing and determining severity of TBI in adults and children.
News
Blood tests taken within 24 hours of a traumatic brain injury (TBI) flag which patients are likely to die and which patients are likely to survive with severe disability, according to a study headed by UC San Francisco, the University of Pennsylvania and the University of Michigan. Their results – available within minutes – may confirm the need for prompt surgical interventions or may help guide conversations with families in cases of devastating injury.
Traumatic Brain Injury: A Roadmap for Accelerating Progress, a new consensus study report from the National Academies of Sciences, Engineering, and Medicine’s Committee on Accelerating Progress in Traumatic Brain Injury Research and Care, examines how progress can be advanced in TBI care and research and develops a roadmap to help guide the field.